Our Helicon™ therapeutics are alpha-helically locked peptides that are mainly comprised of custom non-canonical amino acids. These “large small molecules” are a new drug modality and have required the establishment of an entirely new range of discovery capabilities.
Our platform: A seamless integration of AI and experimental technologies
Chemical and Biological Science
We have developed a large set of non-canonical amino acids and have hundreds of proprietary linkers for stabilizing peptides into an alpha-helical conformation. Combined, these provide us with access to unprecedented chemical diversity, allowing us to finely tune the properties of our Helicons to match the profile needed for each new medicine. To take full advantage of this diversity, we have developed a suite of custom experimental systems that can generate vast quantities of data points across a range of Helicon properties, which both provides input for the construction of AI models and also serves as a high-throughput platform for empirically testing model predictions. We have built substantial know-how with our platforms that allow us to accelerate our discovery programs. And with every program, we rapidly iterate and refine our experimental and AI methods.
AI and Data Science
We leverage state-of-the-art AI- and physics-based computational methods, informed by our unparalleled dataset of non-canonical amino acids and their properties, to drive Helicon design and optimization. In parallel, we employ cutting-edge AI and data science techniques to inform target identification and clinical development by combining both in-house generated data — such as experimental results and proprietary biological insights — with publicly available biological and patient datasets. By combining advanced AI, robust computational methods, and a continuous learning system built on our growing data repository, we are creating a transformative platform that not only accelerates drug discovery but also increases the precision and success of our therapeutic programs.
Therapeutic Hypothesis
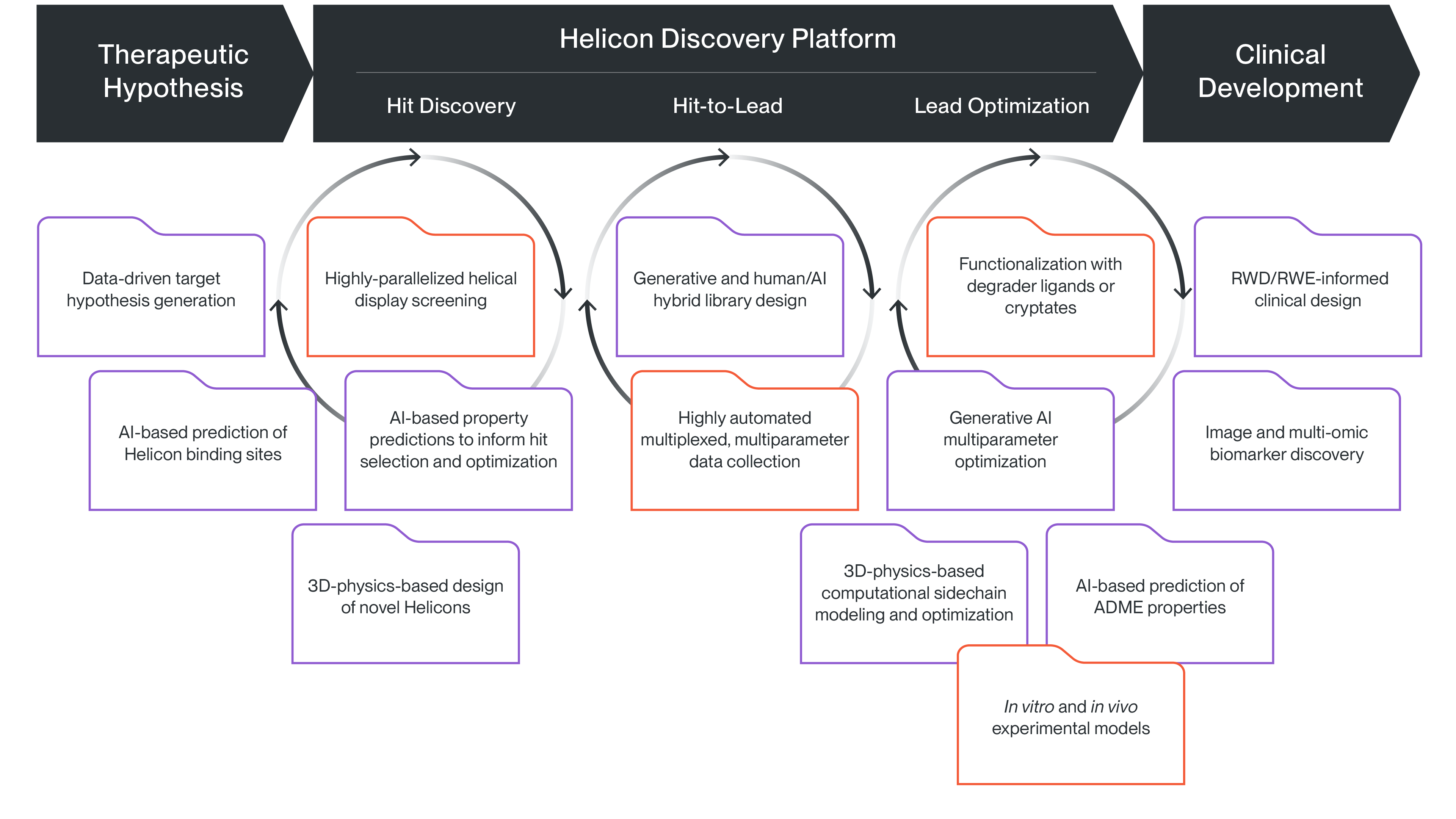
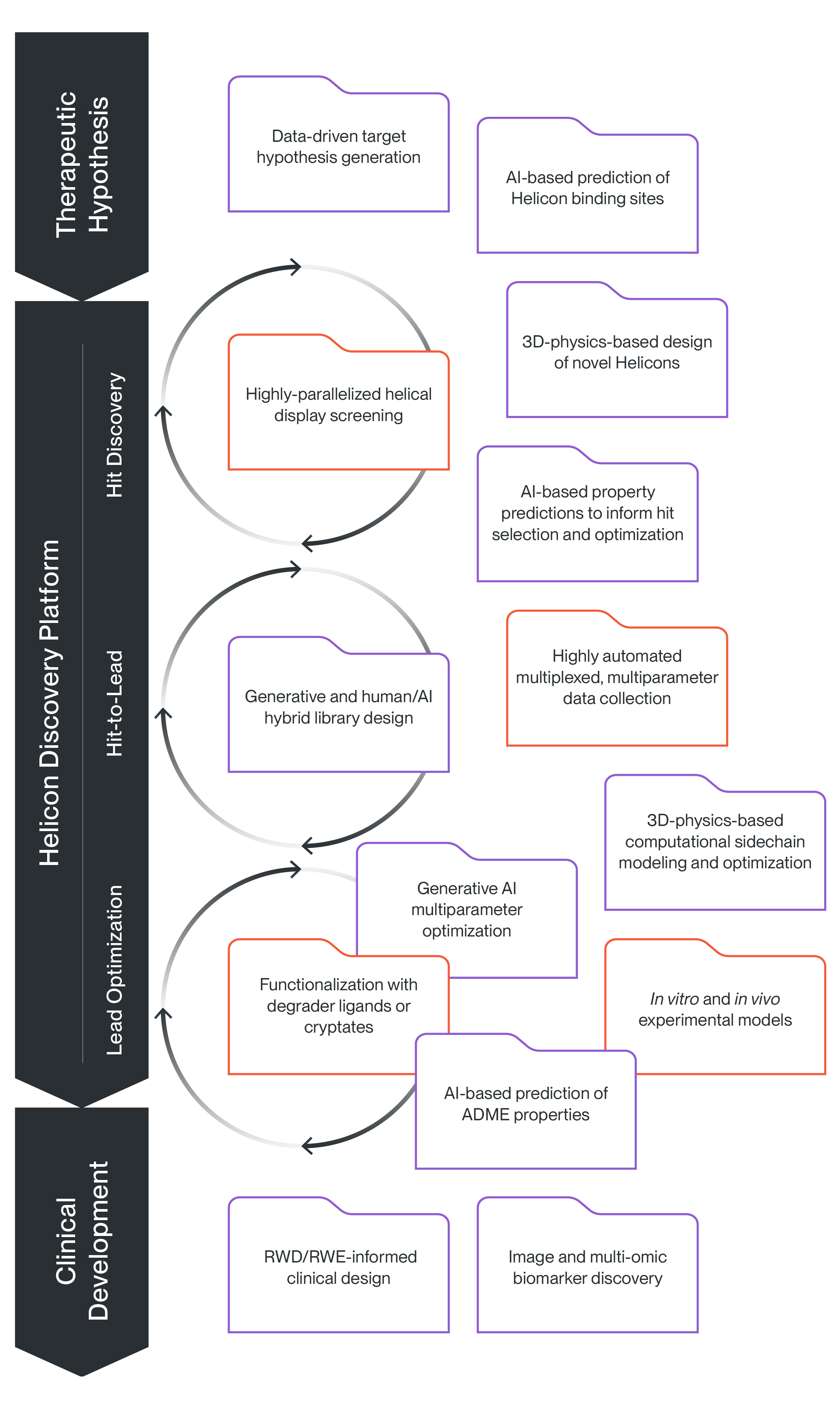
We leverage data science and AI alongside experimental work in the lab to develop high-quality therapeutic hypotheses with an increased probability of patient benefit. Our approach integrates a comprehensive knowledge base that incorporates both publicly available and proprietary biological data, including AI-generated predictions of Helicon binding sites, with clinical insights and patient impact data. By combining computational predictions with experimental validation, we ensure that our hypotheses are not only scientifically sound but also have the potential to translate into meaningful clinical outcomes for patients.
Helicon Discovery Platform
We are able to systematically and rapidly discover Helicons and optimize their therapeutic potential by seamlessly integrating custom and highly automated laboratory-based technologies with cutting-edge AI and other computational algorithms. Our multiplexed display screening and co-optimization platforms power our rapid, efficient exploration of chemical space, and our proprietary cytosolic exposure assays allow us to measure and overcome one of the biggest challenges in intracellular peptide discovery, which is how to optimize their cellular entry and access to the targets. To learn more about how our platform is used to solve different challenges during the drug discovery process, explore the use cases below.
Modular Chemistry, Astronomical Diversity
Multiplexed Experimental Platforms
AI- and Physics-based Computation
Optimized
Properties
Our range of proprietary Helicon crosslinking systems lock peptides in an alpha-helical conformation to facilitate cellular penetration
Custom cell-based assays allow us to quantify cytosolic exposure for thousands of Helicons in a single experiment
High-accuracy AI models built with the cytosolic exposure data can predict cell penetration for new Helicon designs
Helicons with efficient cell entry are able to engage the target protein in cells and in vivo
We have built an internal library of more than a thousand custom amino acids that cover a wide range of shapes, sizes, and properties
Highly automated mass spectrometry-based multiplexed screening platforms generate massive datasets for affinity, physical properties, and DMPK
Generative AI- guided multiparameter optimization is performed using AI models built with the multiplexed screening data
Helicons can be designed to achieve a delicate balancing act between all the key properties the drug will eventually need
Our large collection of E3 linkers and ligands are assembled using modular chemistry to rapidly generate highly diverse degrader libraries
High-throughput measurement of degrader activity is complemented by structural characterization with crystallography and cryo-EM to generate a detailed picture of degrader structure-activity relationships (SAR)
Predictive degradation models and physics-based simulations identify optimal ligands and linker positioning
Helicon degraders achieve deep and durable target degradation in vivo
Diverse linkers and flexible attachment points provide significant optionality for incorporation of lead-212 binding chelators
Multiplexed in vitro and in vivo screening for stability and tissue exposure allow us to finely map the structure-activity relationships that affect distribution
Experimental data are used to enable generative AI- guided optimization of high target affinity and selectivity with distribution and clearance to match the half-life of lead-212
Helicon-enabled alpha radioligand therapeutics (HEARTs) achieve highly selective distribution to tumor cells while avoiding key healthy tissues
Clinical Development
Our platform plays a pivotal role in advancing clinical development by integrating real-world data (RWD) to enhance the understanding of our clinical outcomes. In our clinical trials we are using RWD to contextualize single-arm trial data, providing a more comprehensive understanding of the clinical effect and benchmarking against existing interventions. Additionally, we are collecting and leveraging AI and data science for vast multi-modal datasets, including -omics and imaging data from both patient-derived xenograft (PDX) models and patient samples. These data can help identify potential biomarkers, assess patient benefit, and refine therapeutic hypotheses. By continuously learning from these datasets, our platform can help optimize trial design, guide future clinical strategies, and ultimately improve patient outcomes.

“Helicons exist at a unique intersection of the physical and life sciences: they can be assembled and decoded with methods such as solid-phase synthesis and mass spectrometry, but also with biological techniques such as display screening and deep sequencing. This has allowed us to build a diverse range of experimental discovery technologies that generate large, information-rich datasets, which we have tightly integrated with our AI- and physics-based discovery platforms.”
John McGee, Ph.D. Scientific Co-Founder and Senior Vice President, Platform Technology
